From the journals: February 2019
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and ͵��͵�� & Cellular Proteomics.
Cleaning up light-sensitive debris in the eye
The receptor tyrosine kinase Mer is expressed in the retinal pigment epithelium, where it plays a key role in cleaning up debris from photoreceptor cells through phagocytosis. Mutations in the gene encoding Mer are associated with progressive degeneration of photoreceptor cells, resulting in blindness. It was unknown exactly how the accumulation of debris leads to photoreceptor cell degeneration. In published in the Journal of Biological Chemistry, Jin Zhao and colleagues at Columbia University Medical Center showed that the unphagocytosed photoreceptor cell debris in Mer knockout mice contains bisretinoid fluorophores. These photoreactive molecules appeared to be responsible for light-induced degeneration of the photoreceptor cells of these mice.
Finding protein-coding genes in rice
Did you know that studying the protein content of tissues from the rice plant, Oryza sativa, can help us gain a better understanding of the plant’s genome? In a recent collaborative study between BGI, the University of Liverpool and Baylor College of Medicine, Zhe Ren and colleagues combined transcriptomics and proteomics analyses of rice to find novel genes and splicing events.
In most eukaryotes, a gene’s DNA template is processed through RNA splicing, a process that involves removing intervening sequences (introns) and forming the mature messenger RNA through splicing of exons. The use of high-throughput technologies such as RNA-seq and next-generation sequencing has led to discovery of more exon splicing patterns and protein-coding gene sets. However, these genome-wide approaches suffer from some mistaken annotation and low-level data on thousands of genes or genomic regions.
In published in the journal ͵��͵�� & Cellular Proteomics, the researchers collected public proteomics, genomics and transcriptomics data to discover approximately 100 new protein-coding genes in rice.
Novel enzymes from hot spring bacteria
Extreme environments are often home to unique enzymes. One way to discover new enzymes in these environments is by using functional metagenomics, a culture-independent technique that allows enzymes to be identified by activity rather than by homology to known enzymes. Léa Chuzel and colleagues at New England Biolabs used functional metagenomics to investigate sialidases in bacteria adapted to hot springs. They found a novel sialidase that defined a new glycoside hydrolase family, which catalyzed sialic acid hydrolysis via an inverting reaction mechanism. was published in the Journal of Biological Chemistry.
A pathway for lipid metabolism and antiviral action
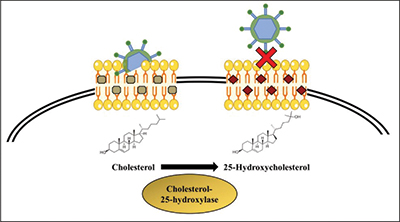 25-hydroxycholesterol inhibits viral infection by blocking the virus from entering the target cell. Gomes et al./BBA The human body possesses a variety of weapons to defend itself against viral infections. For instance, some molecules block viruses from entering cells, and others interfere with viral replication. Understanding these natural responses to infection may be key to developing new antiviral drugs.
25-hydroxycholesterol inhibits viral infection by blocking the virus from entering the target cell. Gomes et al./BBA The human body possesses a variety of weapons to defend itself against viral infections. For instance, some molecules block viruses from entering cells, and others interfere with viral replication. Understanding these natural responses to infection may be key to developing new antiviral drugs.
Previous studies have shown that the molecule 25-hydroxycholesterol, or 25HC, which regulates lipid and cholesterol metabolism, is also a potent inhibitor of viral infection. Production of 25HC is catalyzed by the enzyme cholesterol 25-hydroxylase, or CH25H. Although 25HC has been recognized for its antiviral functions, the signaling pathways that regulate CH25H expression and 25HC levels are not well understood. In published in the Journal of Lipid Research, Ying Liu and colleagues from the Hefei University of Technology in China examined the pathways connecting the antiviral actions of 25HC and activation of the liver X receptor, or LXR. Their research aims to understand the mechanisms involved in 25HC production and metabolism that impact its antiviral activity.
LXR is a transcription factor involved in a number of biological processes, such as lipid metabolism. After binding to a ligand, LXR forms a complex with another receptor and interacts with specific DNA elements to promote expression of target genes. Both 25HC and synthetic LXR ligand can interact with LXR to signal transcription of the CH25H gene. This study showed that binding of 25HC or a synthetic ligand to LXR inhibited certain viral infections, such as herpes simplex virus type 1, in cell culture.
To test their findings in a physiological model, the researchers treated mice with a synthetic LXR ligand and found that viral growth was reduced. Overall, these studies suggest that the LXR signaling pathway may be important for regulating 25HC levels and its antiviral actions. In the future, this pathway may provide a target for antiviral therapeutic development.
— Kerri Beth Slaughter
Treating high cholesterol with antibodies
Hypercholesterolemia is a common medical condition characterized by high levels of cholesterol in the blood. Many patients with the disorder are treated with statins and ezetimibe to lower cholesterol levels. Statins lower cholesterol levels by inhibiting cholesterol synthesis, whereas ezetimibe inhibits absorption of cholesterol by the small intestine. In addition, treatment with antibodies to proprotein convertase subtilisin/kexin type 9, or PCSK9, can be used in combination with these medications to help patients reach target cholesterol levels. Recent clinical trials have shown that two approved PCSK9-antibodies reduced low-density lipoprotein, or LDL, cholesterol and the risk of cardiovascular events.
In in the Journal of Lipid Research, Günther Silbernagel and colleagues at the Medical University of Graz in Austria write that they analyzed 245 patients with hypercholesterolemia to study the effects of PCSK9 antibodies on PCSK9 metabolism, cholesterol synthesis and cholesterol absorption. Results of the clinical trial showed that treatment with statins and ezetimibe led to increased PCSK9 in the blood. Additional treatment with PCSK9 antibodies helped decrease LDL-cholesterol levels without significantly affecting the balance between cholesterol synthesis and absorption. These findings are important for understanding how combinations of cholesterol-lowering treatments affect patients with hypercholesterolemia.
Precise portraits of prions
Normal prion protein can misfold and form pathological aggregates, but the high-resolution structure of these aggregates and why some are infective and some are not remain unknown. To begin to address these fundamental questions, Qiuye Li and colleagues at Case Western Reserve University characterized synthetic prion protein aggregates that were generated under the same conditions but possessed different levels of infectivity in vivo. Using hydrogen-deuterium exchange and hydroxyl radical footprinting, they found that noninfectious aggregates had a less ordered structure and differed in other key respects from infectious prions. was published in the Journal of Biological Chemistry.
Angling for Angelman mechanisms
Angelman syndrome, a genetic condition affecting mental development and speech, results from loss-of-function mutations in the UBE3A ubiquitin ligase gene. Overexpression of this gene, meanwhile, is linked to autism spectrum disorders. In published in the Journal of Biological Chemistry, Simone Kühnle and colleagues at Harvard Medical School characterized UBE3A’s interactions with the 26S proteasome. They found that Angelman syndrome–associated mutations, which did not affect UBE3A’s ubiquitin ligase activity, impaired the enzyme’s ability to bind to the proteasome. As a result, Wnt/beta-catenin signaling was inhibited. These results implicate this signaling pathway in Angelman syndrome.
Protein structure and function in Arabidopsis
 Arabidopsis thaliana, commonly known as mouse-ear cress, is a small flowering weed that grows along roadsides in Eurasia and Africa.Karl Hauser/NIH FlickrHave you ever wondered how some plants in temperate zones survive all four seasons? Unlike many animal species, plants can’t control their internal temperature. Instead, they must survive in ambient conditions. Researchers studying this resilience in fluctuating temperatures have found an answer within plants’ proteins.
Arabidopsis thaliana, commonly known as mouse-ear cress, is a small flowering weed that grows along roadsides in Eurasia and Africa.Karl Hauser/NIH FlickrHave you ever wondered how some plants in temperate zones survive all four seasons? Unlike many animal species, plants can’t control their internal temperature. Instead, they must survive in ambient conditions. Researchers studying this resilience in fluctuating temperatures have found an answer within plants’ proteins.
In the journal ͵��͵�� & Cellular Proteomics, Jeremy Volkening and colleagues at the University of Wisconsin-Madison on the thermal proteome of Arabidopsis thaliana, commonly known as mouse-ear cress.
Thermal proteome profiling, or TPP, is the use of high-throughput mass spectrometry–based quantitation to look at thousands of proteins’ melting temperatures simultaneously. Before this method was established, researchers had to go through the laborious isolation of one purified protein at a time to look at physical or chemical properties. The technique takes advantage of the fact that denatured proteins tend to aggregate and precipitate out of solution. By tagging each sample in a temperature gradient with a unique isobaric tag, the amount of each protein remaining in aqueous solution at each temperature can be compared using a technique known as tandem mass tag labeling. Melting temperature of proteins can be used to study the proteins’ structure and their ability to maintain their native conformation under increasing temperature — a property called thermostability.
The researchers found substantial variability among proteins; some reproducibly displayed the same melting temperature and others had high variability. The authors showed correlations between protein thermostability and other characteristics such as molecular weight. This information provides the groundwork to utilize TPP in future plant studies to fill the gaps in the knowledge of plants’ protein expression, protein-protein interactions, and their binding sites.
— Gelareh Abulwerdi
What can we learn from the maize genome?
Before it was domesticated, the ancestor to maize was a grass species called teosinte found mainly in Mexico’s tropical southwestern region. As it was domesticated, it not only increased in productivity but also spread to more temperate climates, where day length, temperature and local diseases were dramatically different. How did maize adapt so well to varying climates?
In collaboration with the Beijing Institute of Genomics, Hubei Collaborative Innovation Center for Grain Industry, and China Agricultural University, Lu-Guang of China Agricultural University published in the journal ͵��͵�� & Cellular Proteomics describing the relationship between mRNA and protein levels during this adaptation from tropical to temperate climate by comparing 98 different strains of maize.
The central dogma of molecular biology is that DNA is transcribed to RNA and RNA is translated into protein. With recent advances in understanding of gene regulation, this process is not as straightforward as it looks. In fact, the abundance of the transcript at the level of RNA does not reflect the expression pattern of proteins due to post-transcriptional events such as alternative splicing, post-translational modifications, protein folding, transport and localization.
The researchers showed that although mRNA abundance affects biological functions, post-transcriptional modifications also play important roles in synchronizing expression regulation toward genes in certain biological pathways.
Why two enzymes evolved to stick together
Aminoacyl-tRNA synthetases, or ARSs, link tRNAs to their corresponding amino acids, and a different ARS carries out this function for each amino acid. In animals, glutamyl-tRNA synthetase and prolyl-tRNA synthetase, which produce the tRNAs carrying glutamine and proline, are fused permanently together as a bifunctional protein known as EPRS. Sandeep M. Eswarappa and colleagues at the Indian Institute of Science investigated what the advantage of fusing these two enzymes together might be by modeling the connection between amino acid availability and ARS expression. They suggest that, since glutamine is a metabolic precursor of proline in the citric acid cycle, forcing the ARSs corresponding to these two amino acids to be expressed simultaneously helps keep the cell from critically depleting glutamine levels when synthesizing proline-rich proteins. was published in the Journal of Biological Chemistry.
Transporting ions or antibiotics
EmrE is an ion-coupled transporter that bacteria use to eject antibiotics out of the cell, enabling antibiotic resistance. A key question about ion-coupled transport is whether ions and substrates are bound simultaneously or competitively exclude each other. In published in the Journal of Biological Chemistry, Nathan E. Thomas and colleagues at the University of Wisconsin and Washington University showed that EmrE binds protons and substrates simultaneously but that substrate binding results in deprotonation of a residue outside of the substrate-binding pocket. This suggests that the mechanism of protein-coupled transport may be more complex than assumed by previous models.
Two studies of one tough spore
 Clostridium difficile bacteriumClostridium difficile is a bacterium that causes severe gastrointestinal infections, especially in hospitals among patients who are immunocompromised or have been taking antibiotics. In 2009, its increasing prevalence in healthcare settings led the World Health Organization to classify it as an emerging infection.
Clostridium difficile bacteriumClostridium difficile is a bacterium that causes severe gastrointestinal infections, especially in hospitals among patients who are immunocompromised or have been taking antibiotics. In 2009, its increasing prevalence in healthcare settings led the World Health Organization to classify it as an emerging infection.
C. difficile is naturally resistant to most broad-spectrum antibiotics, largely due to the tough protective spore coat that it’s able to form. C. difficile spores can be transmitted through the environment and latently persist within the intestine, leading to recurring infections. Two recent papers in the Journal of Biological Chemistry examined the unique biochemistry of C. difficile spore formation, paving the way for development of antibiotics.
In , Emma Richards and colleagues at Imperial College London examined modification to the S-layer, a layer of proteins surrounding the cell wall. They found that some S-layer proteins carried a complex glycan modification. This modification was encoded by a large (24 kilobase) insertion in the genome of a subset of known C. difficile strains. Mutational analysis showed that without this glycan modification, cells were less able to form spores and adhere to intestinal cells. A class of antibiotics in development for C. difficile targets S-layer proteins, and it’s still unknown whether this glycosylation —which only occurs in some C. difficile strains — affects the action of these antibiotics.
In , Héloise Coullon and colleagues at Université Paris-Sud focused on a layer of the C. difficile spore coat called the spore cortex. They found that the composition of the C. difficile spore cortex differed significantly from that of Bacillus subtilis, the most thoroughly studied spore-forming bacterium. They zeroed in on cortex protein modification with glycans called muramic-delta-lactams. Without this modification, C. difficile was less able both to form spores and to germinate out of spores and was more sensitive to heat.
Together, the two studies highlight how glycan modifications characteristic of C. difficile affect the robustness of its spore — an important consideration when developing treatments.
— Sasha Mushegian
Low HDL linked to cardiovascular disease
High-density lipoprotein, or HDL, transports cholesterol and phospholipids through the blood to the liver. The liver is responsible for removing cholesterol from the body or redistributing it to other tissues. Because the main genes that regulate HDL cholesterol level also affect other blood lipids, there is debate in the field as to whether very low HDL levels caused by genetics can independently trigger atherosclerosis and cardiovascular disease.
In published in the Journal of Lipid Research, Andrew Geller and colleagues from the company Boston Heart Diagnostics and Tufts University were the first to analyze the genetic and secondary causes of severe HDL deficiency in a large population to identify links to atherosclerotic cardiovascular disease. Initially, they screened more than 250,000 individuals and selected 308 eligible subjects for further study based on their low levels of HDL cholesterol. DNA from eligible subjects was sequenced to examine lipoprotein metabolism genes for different HDL deficiency states.
Results from this study were consistent with the finding that in about 40 percent of cases, severe HDL deficiency has nongenetic causes such as uncontrolled diabetes and increased inflammation. About half of the low HDL cholesterol patients without these causes had mutations or variants in lipoprotein metabolism genes such as ABCA1, LCAT or APOAI. Contrary to other published work that omitted those major genes, results of this study suggested that genetic causes of severe HDL deficiency can lead to premature atherosclerotic cardiovascular disease.
Upregulating antioxidants in the brain
Oxidative stress is thought to be a trigger for Alzheimer’s disease, and the Alzheimer’s disease brain has diminished antioxidant activity. Reducing oxidative stress in the brain therefore could be a strategy for slowing neurodegeneration, but there are few global antioxidants that can permeate the blood-brain barrier, or BBB. In published in the Journal of Biological Chemistry, Kelsey Murphy and colleagues at the University of Toledo developed a BBB-permeable activator of Nrf2, a transcription factor for the majority of antioxidant enzymes. This activator, a polysaccharide called mini-GAGR that is administered through the nose, decreased brain mitochondrial oxidative stress and improved memory in mice.
Packing DNA for long distances
DNA is packed efficiently into histone-based nucleosomes, which then are condensed into chromatin. The structure of chromatin determines the accessibility of genes to transcription. Laxmi Narayan Mishra and Jeffrey J. Hayes from the University of Rochester investigated how histone presence, histone acetylation and the presence of nucleosome-free regions affected the accessibility of DNA to DNA-binding proteins. They found that the effects of histones and nucleosome-free regions affected DNA accessibility beyond the immediate site, suggesting mechanisms of gene regulation via higher-order folding effects on chromatin. was published in the Journal of Biological Chemistry.
Insights into cytochrome P450
Cytochrome P450 1A1, or CYP1A1, can detoxify drugs and environmental toxins but also can produce carcinogenic reactive intermediates. Therefore, selectively activating or inhibiting CYP1A1 could be a strategy to prevent cancer or regulate activity of anti-cancer drugs. Aaron G. Bart and Emily E. Scott from the University of Michigan examined the structural determinants of how CYP1A1 binds to diverse substrates. , published in the Journal of Biological Chemistry, suggest that modifications in the active site allow the enzyme to accommodate large ligands.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Sweet secrets of sperm glycosylation
Scientists from Utrecht University uncover similar glycosylation patterns in sperm from bulls, boars and humans, distinct from those found in blood across species. These findings may improve IVF and farming techniques.

From the Journals: JLR
Promising therapeutic candidate for steatosis. Unique lipid profiles in glycogen storage disease. Microglial lactic acid mediates neuroinflammation. Read about these recent papers.

Meet Robert Helsley
The Journal of Lipid Research junior associate editor studies chronic liver disease and was the first in his family to attend college.

From the Journals: MCP
Protein acetylation helps plants adapt to light. Mapping protein locations in 3D tissues. Demystifying the glycan–protein interactome. Read about these recent papers.

Exploring life’s blueprint: Gene expression in development and evolution
Meet Julia Zeitlinger and David Arnosti — two co-chairs of the ASBMB’s 2025 meeting on gene expression, to be held June 26-29, in Kansas City, Missouri.

From the journals: JLR
Protein analysis of dopaminergic neurons. Predicting immunotherapy responses in lung cancer. ZASP: An efficient proteomics sample prep method. Read about papers on these topics recently published in ͵��͵�� & Cellular Proteomics.



.jpg?lang=en-US&width=300&height=300&ext=.jpg)