Making O
The blood industry is in a bind.
Over the past decade, the amount of blood transfused in the United States has plummeted due to improved patient management and strategic scheduling of elective surgeries. While this has cut costs for hospitals, insurance providers and patients, it has upended the nonprofit business model of blood suppliers in the United States.
In August, the American Red Cross — the country’s largest blood supplier, providing around 40 percent of the blood used in hospitals — that it would need to raise the price of the blood it sells to hospitals to cover its operating costs. According to Red Cross officials, they already have reduced staff and donor-recruitment efforts. Experts say all this does not bode well for the industry as a whole.
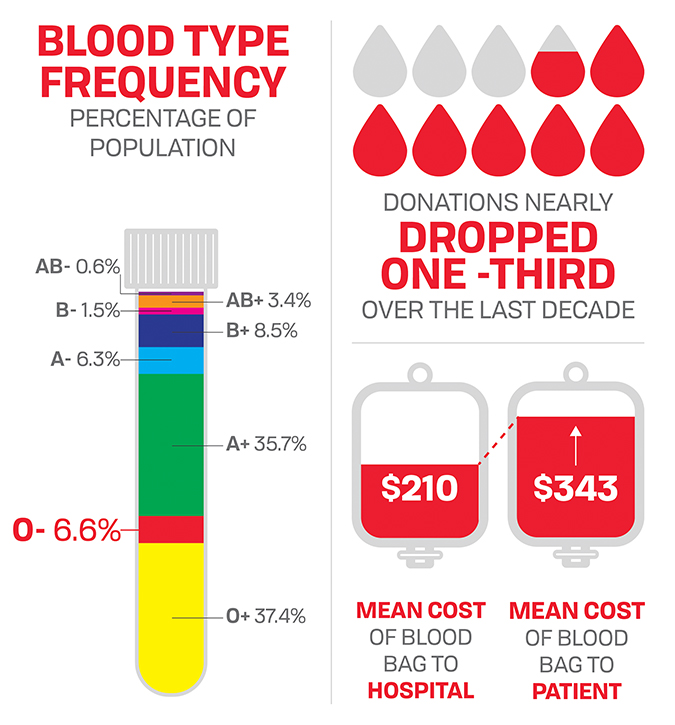
Meanwhile, blood suppliers face a near-constant shortage of universally acceptable O-negative blood. Only of the U.S. population has Type O-negative, which is used by hospitals in almost all emergency surgeries and traumas that require transfusions.
One step toward addressing the shortage of O-negative blood may be to convert other types of blood to O. Recent work by researchers at the University of British Columbia using enzymes from human gut bacteria to modify A and B red blood cells may bring us closer to the solution.
A quest for enzymes
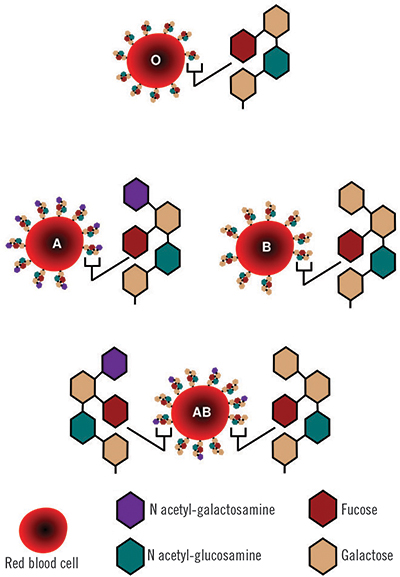
Human red blood cells come in four major types — A, B, AB and O — based on the presence or absence of A and B antigens on the cell surface. The A antigens cause a person’s immune system to produce antibodies against the B antigens, and vice versa, meaning that a person with Type A blood will have a potentially fatal reaction to a transfusion of B red blood cells.
In addition, each blood type comes in two subtypes based on the presence or absence of a protein antigen called the Rh factor. A transfusion of blood with a positive Rh factor will generate an immune response in a person who is Rh negative, but the reverse is not true. Among the eight blood types determined by antigens and Rh, O-negative red blood cells lack the A, B and Rh antigens and can be donated to a person with any blood type. Conversely, AB-positive blood can be donated only to patients with the same blood type, and patients with AB-positive blood are able to receive a transfusion of any blood type, because their immune systems do not generate antibodies to any of the antigens.
Beyond these primary eight types, blood comes in more than 600 subtypes, which come into play when transfusions are needed for people with diseases such as sickle cell anemia.
The A and B antigens differ from one another by a single sugar: where the A antigen has N-acetylgalactosamine, the B antigen has galactose. Scientists have been attempting to snip those sugars off A and B red blood cells enzymatically for decades with the goal of creating blood cells with antigens that closely resemble the base antigen found on O red blood cells.
In 1982, a group of scientists led by Jack Goldstein at the New York Blood Center’s Kimball Research Institute in the journal Science that enzymes isolated from green coffee beans could remove the galactose from B blood cells, functionally converting them to O blood cells.
In 1991, the medical device company , based outside Boston in Beverly, Massachusetts, partnered with Goldstein, intending to create a system that could convert large quantities of blood from B to O. Mechanical engineers at ZymeQuest developed a and software for washing, converting and repackaging red blood cells. However, the enzymes were slow and inefficient. Goldstein died in the late ’90s, before his team at the New York Blood Center could find an enzyme that could remove GalNAc from Type A red blood cells.

In 2000, hematologist Thomas P. Stossel joined ZymeQuest’s . “The bad news was that B is not as common as A, and so converting B red blood cells wasn’t really a viable commercial exercise,” explained Stossel, recently retired from Brigham and Women’s Hospital in Boston. “You really needed to be able to get the A, and the A just didn’t work with the coffee bean stuff.”
In , Henrik Clausen, a biochemist at the University of Copenhagen and consultant to ZymeQuest, in the journal Nature Biotechnology that a set of novel glycosidases could remove the sugars on the antigens on both Type A and Type B blood cells.
“So then came (Clausen), who was a carbohydrate maven,” Stossel said. “(He) worked on it and ostensibly seemed to have come up with a conversion process.”
The company continued to test Clausen’s enzymes for a few years with disappointing results, Stossel said. The enzyme-treated Type-A cells registered as Type O in serologic tests but did not circulate when infused into test subjects.

According to William Skillman, who came aboard as the company’s chief executive officer in 2014, the failure of Clausen’s enzymes to perform as hoped began to draw the long project to a close.
“The investment to take it forward … probably would have been another $30 million or more,” Skillman said. “Investors were tired, and I think that was the end of the game.”
In , ZymeQuest, by then renamed Velico Medical, sold some of its royalty rights to the universal blood platform and shifted focus to developing a process for spray-drying plasma and storing it at room temperature, as well as an improved medium for platelets.
Dollars and deciliters
Blood donations began in earnest during World War II in England, and the industry that emerged steadily grew over the next six decades.
By , hospitals in the U.S. transfused an 15 million bags of blood per year, according to the Department of Health and Human Services’ biennial National Blood Collection and Utilization Survey. This was a slight uptick from the 14.65 million bags transfused in , 14.18 million in and an estimated 13.9 million in 2001.

The average price a hospital paid for a bag of blood was $223 in 2008, up from $143 in 2001. It increased slightly in 2011 to $225 and held steady through 2013, according to the HHS.
The biennial surveys do not include information about how much hospitals charge patients for blood. But according to a Thomas Jefferson University in 2011, hospital patients were charged $343 on average for a bag of blood in 2007, when hospitals paid suppliers $210.
For decades, patients routinely received blood transfusions during nonemergency surgeries when their blood levels of hemoglobin, the iron-based protein in red blood cells that binds to oxygen, fell below 10 grams per deciliter.
This threshold, set in 1942, wasn’t scientifically challenged until 1994, when, concerned about the burden of testing blood for HIV and hepatitis, a group of researchers in Canada questioned if patients would do better if they changed the hemoglobin standards for giving transfusions.
The researchers studied 838 intensive-care patients randomly assigned to receive a transfusion when their blood hemoglobin levels fell below either 7 or 10 grams per deciliter. The study, published in , found that after 30 days, the people in the group with the lower threshold had received an average of three fewer units of blood than group with the higher threshold but had the same probability of death.
Over the next decade, more studies provided evidence that hospitals could decrease blood transfusions without putting patients at risk.
A in the New England Journal of Medicine reported that lowering the hemoglobin threshold in stable, critically ill children to 7 grams per deciliter didn’t increase adverse outcomes.
In , Stanford Medical Center began a study that used the hospital’s electronic records program to alert doctors of transfusion guidelines when they requested transfusions for patients with hemoglobin levels above 8 grams per deciliter.
The study, results of which were published in and in the journal Transfusion, found that the alerts reduced red blood cell use by 24 percent from 2009 to 2012, which saved the hospital an estimated $1.6 million.
More significantly, the drop in transfusions corresponded with a two percent reduction in mortality rate among people who had transfusions, from , for reasons that weren’t entirely clear.
In 2012, the American Medical Association held a summit on overuse of five medical interventions, including transfusions of red blood cells, and the American Association of Blood Banks put out recommending that transfusions be given to stable patients only when their hemoglobin levels fell below 7 grams per deciliter.
Separate studies at multiple hospitals found that transfusing blood at lower hemoglobin thresholds for patients undergoing , , , and had the same effect on patient outcomes as transfusing blood at higher thresholds. However, , more liberal transfusion strategies were found to provide better outcomes for patients undergoing surgery for cancer.

According to , chief scientist of the Canadian Blood Services, these studies had an impact across hospital transfusion departments and the hematology community at large.
“We want to only give blood if you need to,” Devine said, and doctors can avoid unnecessary transfusions by monitoring patients’ blood iron levels before surgery and making sure they’re in the best possible condition.
“This has been warmly embraced, not only by treating physicians but also by insurance companies who have to pay for blood products,” Devine said, adding that patient blood-management programs started by hospitals in the last 10 years have caused demand for blood products in the U.S. to drop by at least a third.
This drop is largely in transfusions during elective surgeries, when the transfused blood would be matched to the patient’s blood type. But O-negative blood, which is used in emergency surgeries when there isn’t time to determine a patient’s blood type, is still in constant demand.

Gut reactions
After ZymeQuest bowed out of developing a platform to create universal blood in 2010, reports of research into enzymes that could convert A and B blood largely dried up until 2015.
That year, and a collaborator at the University of British Columbia’s Centre for Blood Research, , published findings in the Journal of the American Chemical Society about enzymes that could digest the sugars off of A and B blood. According to Withers, an enzymologist who earned his Ph.D. from the University of Bristol in 1977 and began working at UBC in 1982, his was inspired by Clausen’s 2007 paper in Nature Biotechnology.
“In that 2015 paper, we had taken the tack that there was an existing enzyme … which could be used to convert A or B blood to something like O. It’s not exactly O,” Withers said.
While the researchers successfully used directed evolution to make an enzyme that could cleave the GalNAc from a single subtype of A blood, making it resemble O blood, the enzyme didn’t work against every subtype of blood within that group.
“We came to realize that this was going to be a pretty long haul if we were going to do this for each of the subtypes,” Withers said. “So I thought maybe it’s time to take a step back and see if there’s a better start point for this. Maybe we can find an enzyme in nature already, using metagenomics.”
Withers had begun collaborating with , a metagenomics researcher at UBC. At first, the two considered focusing on blood-drinking creatures that might harbor sugar-eroding enzymes.
“You’d tend to think of the guts of mosquitoes, leeches, vampire bats and things like that,” Withers said. “But these had to be beasties that are (exclusively) feeding on humans, because it’s only realistically humans that have the ABO blood system.”
So the biochemists turned their thoughts inward, to the trillions of bacteria that make up the human microbiome. The walls of our intestines are lined with a layer of mucin sugars that serve the double duty of protecting our gut from the bacteria that call it home and preventing those bacteria from being swept away. Withers reasoned that, since some of the bacteria had evolved to digest both this carbohydrate surface and other sugars, some of them might also have evolved to digest the sugars on human blood cells.
“It’s an unpleasant but relatively convenient source of materials,” Withers said. “Of course, we’re not opening up humans, we’re just collecting feces.”
Withers and his colleagues didn’t know if there was a connection between blood type and microbiome content, so they hedged their bets and had a colleague with AB blood who hadn’t taken antibiotics in the last month donate stool samples.
What followed was standard metagenomics: the researchers lysed the bacteria from the stool samples, extracted their DNA and sliced the DNA into 30- to 50-kilobase chunks.
Rather than sequence all of the DNA chunks at this step, the researchers next used phages to insert the DNA into Escherichia coli. They then grew and lysed the E. coli and used a unique assay to test which had gained the ability to chew the sugars off red blood cells.
Once they had a hit, they grew the bacteria of interest and sequenced the corresponding gene inserts to determine if any novel enzymes from the DNA chunks were responsible for the new digestive capabilities.
“You’re hoping that you don’t see anything you recognize in there,” Withers said. “That’s what we were able to do in this case, get a new enzyme family.”
To their delight, that family, isolated from gut microbes and encoded by E. coli, consisted of hundreds of new enzymes, which they hoped would overcome some of the shortcomings of the enzymes Clausen had identified in 2007.
“Their enzymes would not work in whole blood,” Withers said of Clausen’s work. “They had to be separated red blood cells in a low ionic-strength buffer to do this conversion. We were looking for something that’s a little more convenient and a little more active. Indeed, the one we found will work in whole blood, and, depending exactly how you measure these things, it’s something like 30 times more active than theirs.”
According to the Canadian Blood Service’s Devine, who is also a professor of pathology and laboratory medicine at UBC, one problem ZymeQuest faced in adapting Goldstein’s enzymes isolated in 1982 into a universal blood platform was their relatively low reaction rate. This meant the system needed high volumes of the enzyme, which drove up the speculative cost of the system.
“Steve’s managed (to) make it so that you can add much less enzyme than was originally in the ZymeQuest treatment,” Devine said. “So, in theory, that means you should be able to drop the major cost component in the treatment.”
After Withers and colleagues tested a number of their enzymes against synthetic versions of the sugars on red blood cells to narrow their focus, they examined the activity of a subset of the enzymes on red blood cells. When they then treated whole Type A blood with these enzymes and ran it through standard typing kits provided by the Canadian Blood Services, the blood successfully registered as Type O.
Bad for business
The drop in demand for any blood that is not O-negative has coincided with a decline in donations.
According to the Centers for Disease Control and Prevention’s Office of Blood, Organ and Tissue Safety, the number of units collected for transfusion dropped to 12.2 million in 2017 from 17.3 million in 2009
The price of blood also has declined. Hospitals paid a median price of $207 for each bag of red blood cells in 2017, $14 less than in 2013.
According to Devine, the fall in blood donations, demand and prices, and the shortage of O-negative blood, aren’t uniquely American problems.
“The decrease in red cell utilization has happened in all developed countries in reaction to patient blood management,” she said. “So, it’s good for patients, (but) it’s just not so good if you run your blood system like a business.”
The decrease in blood prices and number of transfusions has eaten into the operating budgets of blood providers of all sizes, whose costs largely consist of paying employees to draw, test, process, store and deliver blood, as well as to recruit and track donors.

According to , president of biomedical services at the American Red Cross, his organization and others have had to cut back on staff and curtail blood drives. “Because of the industry economics, we can no longer afford to maintain the spare capacity that’s not productive,” Hrouda said.
In the past, blood centers have been able to respond to temporary surges in donors after events, such as snowstorms or hurricanes, caused regular donations to dip. With reduced staffing, blood banks are now slower to bounce back after disruptions.
Additionally, whole blood donations have decreased across the board, and industry experts aren’t sure why.
“We just find it harder and harder to recruit donors in the United States, and that adds to the cost challenges,” Hrouda said. “It could be … that more potential donors are engaged in the paid plasma industry, where they can go and donate and receive some financial contribution.”
As O-negative blood remains in constant demand, experts say, blood of other types isn’t piling up in refrigerators, collected and unused.

According to , executive vice president of Blood Centers of America, which represents more than 50 independent blood suppliers, the amount of blood that spoils at blood banks doesn’t play a significant role in the costs of blood operators. In 2017, BCA members “outdated” less than 2 percent of the blood they collected.
“What we are seeing though, is that hospitals are requesting and using a lot more O-positive and O-negative,” Salamon said. “There’s been an overabundance in use of O-negative, so I think that is really where a neutral red cell would gain some traction — in hospitals.”
Tight inventory management has pushed blood suppliers in the U.S. to take action beyond cutting staff. Hrouda, who wrote the open letter in August announcing that the Red Cross was raising prices of most blood products, said last month that those increases already have gone into effect.
“We are not going to push our way to prosperity through cuts of people and productivity improvements — by cutting people and just saying ‘Do more. You just need to work harder,’” Hrouda said. “That is never going to be the solution for a sustainable blood industry in the United States, so we have to innovate, we have to use new technologies.”
A natural challenge
As Withers and colleagues finesse the development of enzymatically rendered Type O blood cells, they will eventually have to reckon with the Rh factor, a protein present on 85 percent of all blood that attaches to the cells in a manner completely different from that of antigenic sugars, before being able to generate truly universal blood from any type.
Fifteen percent of the population has Rh negative blood, and almost half of that group (6.6 percent of the population) has O-negative — already universal donor. So if the blood of everyone else who is Rh-negative could be transformed into type O, the amount of universal blood available for transfusion potentially could be more than doubled. Blood suppliers and hospitals certainly would welcome this prospect.
“I think it’s a great thing, if it could come to fruition, because we need more neutral blood cells,” said Salamon at Blood Centers of America. “We need more Os, and if there is a way to get a product out that looks like an O, that would be a great thing for hospitals and blood centers.”
The technology to make that product is still in the future, as basic questions remain about the process.
“At this stage, we know we’ve converted A to O, and we’re just now getting into the safety test of this A-to-O type blood,” Withers said. “Is it normal O-type blood, or have we chopped something else and caused other problems?”
Stossel, the hematologist at Velico Medical, also stressed the risk of underestimating the molecular nuances.
“I suspect that, as much as we understand about the carbohydrate structures that determine these blood types, they’re actually more complicated and subtle,” he said. Chopping off sugars with an enzyme might seem logical and straightforward, he said, but “nature is a bitch.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and weтАЩll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Guiding grocery carts to shape healthy habits
Robert тАЬNateтАЭ Helsley will receive the Walter A. Shaw Young Investigator in Lipid Research Award at the 2025 ASBMB Annual Meeting, April 12тАУ15 in Chicago.

Quantifying how proteins in microbe and host interact
тАЬTo develop better vaccines, we need new methods and a better understanding of the antibody responses that develop in immune individuals,тАЭ author Johan Malmström said.

Leading the charge for gender equity
Nicole Woitowich will receive the ASBMB Emerging Leadership Award at the 2025 ASBMB Annual meeting, April 12тАУ15 in Chicago.

CRISPR gene editing: Moving closer to home
With the first medical therapy approved, thereтАЩs a lot going on in the genome editing field, including the discovery of CRISPR-like DNA-snippers called Fanzors in an odd menagerie of eukaryotic critters.

Finding a missing piece for neurodegenerative disease research
Ursula Jakob and a team at the University of Michigan have found that the molecule polyphosphate could be what scientists call the тАЬmystery densityтАЭ inside fibrils associated with AlzheimerтАЩs, ParkinsonтАЩs and related conditions.

From the journals: JLR
Enzymes as a therapeutic target for liver disease. Role of AMPK in chronic liver disease Zebrafish as a model for retinal dysfunction. Read about the recent JLR papers on these topics.

