JBC: Iron–sulfur cluster research offers new avenues for investigating disease
Many important proteins in the human body need iron-sulfur clusters, tiny structures made of iron and sulfur atoms, to function correctly. Researchers at the National Cancer Institute, the National Institutes of Health and the University of Kentucky have discovered that disruptions in the construction of iron-sulfur clusters can lead to the buildup of fat droplets in certain cells. These findings, published in the Journal of Biological Chemistry, provide clues about the biochemical causes of such conditions as nonalcoholic fatty liver disease and clear-cell renal carcinoma.
“Iron-sulfur clusters are delicate and susceptible to damage within the cell,” said , the postdoctoral fellow who led the new study. “For this reason, the cells in our body are constantly building new iron-sulfur clusters.”
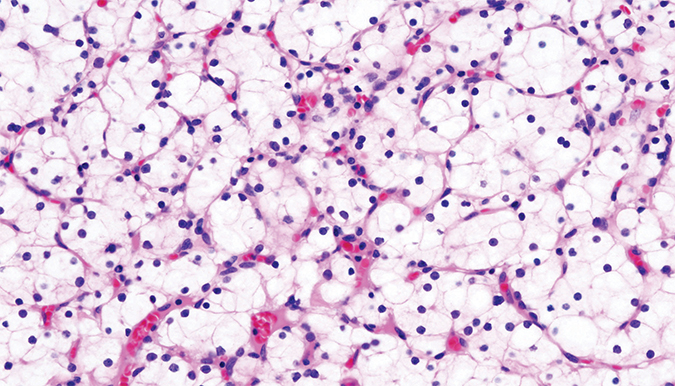 This stained histopathologic image shows clear-cell carcinoma of the kidney from a nephrectomy specimen. The cancer is named for its accumulation of lipid droplets.Courtesy of KGH/Wikimedia Commons Crooks began studying the enzymes that build iron-sulfur clusters during his graduate studies in lab at the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH. Mutations in one of these enzymes can cause ISCU myopathy, a hereditary condition in which patients, despite seeming strong and healthy, cannot exercise for more than a short time without feeling pain and weakness.
This stained histopathologic image shows clear-cell carcinoma of the kidney from a nephrectomy specimen. The cancer is named for its accumulation of lipid droplets.Courtesy of KGH/Wikimedia Commons Crooks began studying the enzymes that build iron-sulfur clusters during his graduate studies in lab at the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH. Mutations in one of these enzymes can cause ISCU myopathy, a hereditary condition in which patients, despite seeming strong and healthy, cannot exercise for more than a short time without feeling pain and weakness.
It was clear to Crooks that lifelong deficiency of iron-sulfur clusters caused profound changes in how cells processed energy. But he wondered exactly what happened in a cell in the first moments after something went wrong with iron-sulfur cluster production. Which of the many proteins that need iron-sulfur clusters were affected first, and what effect did this have on cell metabolism?
Crooks developed experimental methods to abruptly stop iron-sulfur clusters from being manufactured in cells and to monitor what happens to how these cells process glucose. Ordinarily, over a series of metabolic steps, cells would convert glucose into energy. But without iron-sulfur clusters, an enzyme called aconitase that carries out one of the steps in this process doesn’t work. As a result, the cells quickly accumulated an intermediate metabolic product called citrate, which eventually was converted into droplets of fat.
Over-accumulation of fats in tissues where they’re not normally found is a hallmark of numerous diseases, including nonalcoholic fatty liver disease, a risk factor for cirrhosis and liver cancer. suggest that this state could be caused by failures of iron-sulfur cluster production — for example due to cellular stressors or toxin exposure.
“We’re hoping that the people who are working so hard on nonalcoholic fatty liver disease will find our paper helpful to their research,” Rouault said.
Crooks, working in the laboratory of surgeon and scientist at the NCI, is now examining the role of iron-sulfur cluster formation and aconitase function in cancers such as clear-cell carcinomas. Various cancers often are characterized by excessive fat accumulation in cells. In fact, this accumulation of lipid droplets is where clear-cell carcinomas get their name: when a slice of such a tumor is fixed on a slide and its proteins are stained, the large areas of lipid accumulation in the cells look transparent.
“We really want to look at the beginnings of cancer,” Crooks said, “… to understand whether the lipids were formed from glucose or other fuels and whether the lipids are important for pathogenesis, or whether they’re just bystanders that form in response to metabolic reprogramming, which is likely to include disruption of iron sulfur protein activities.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Guiding grocery carts to shape healthy habits
Robert “Nate” Helsley will receive the Walter A. Shaw Young Investigator in Lipid Research Award at the 2025 ASBMB Annual Meeting, April 12–15 in Chicago.

Quantifying how proteins in microbe and host interact
“To develop better vaccines, we need new methods and a better understanding of the antibody responses that develop in immune individuals,” author Johan Malmström said.

Leading the charge for gender equity
Nicole Woitowich will receive the ASBMB Emerging Leadership Award at the 2025 ASBMB Annual meeting, April 12–15 in Chicago.

CRISPR gene editing: Moving closer to home
With the first medical therapy approved, there’s a lot going on in the genome editing field, including the discovery of CRISPR-like DNA-snippers called Fanzors in an odd menagerie of eukaryotic critters.

Finding a missing piece for neurodegenerative disease research
Ursula Jakob and a team at the University of Michigan have found that the molecule polyphosphate could be what scientists call the “mystery density” inside fibrils associated with Alzheimer’s, Parkinson’s and related conditions.

From the journals: JLR
Enzymes as a therapeutic target for liver disease. Role of AMPK in chronic liver disease Zebrafish as a model for retinal dysfunction. Read about the recent JLR papers on these topics.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)