Unexpected roles of lipid kinases
Phospholipids are not mere structural components of animal cell membranes. Many of their metabolites are physiologically functional and are pivotal in intracellular signaling. Of those, diacylglycerol, or DAG, is a second messenger activating proteins containing a C1 domain, such as protein kinase C.
Evidence shows that phorbol esters, synthetic functional analogues of DAG, constitutively activate signal transduction pathways that lead to tumor formation. Thus, normal cells must regulate DAG levels within the physiological range. DAG kinases, or DGKs, are the key enzymes that regulate DAG levels. DGK phosphorylates DAG to phosphatidic acid, another lipid messenger molecule. DGKs are thought to be involved in the balanced control of these two lipid messenger signaling systems.
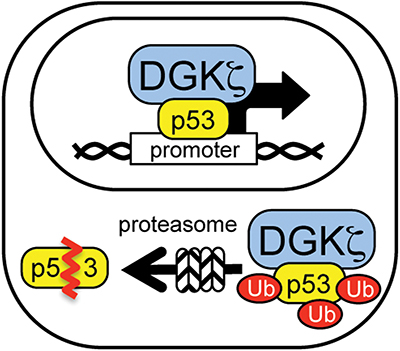 Functional implications of DGK-zeta in p53 regulation: Nuclear DGK-zeta modulates p53 transcriptional activity; cytoplasmic DGK-zeta facilitates p53 protein degradation.Courtesy of Kaoru Goto
Functional implications of DGK-zeta in p53 regulation: Nuclear DGK-zeta modulates p53 transcriptional activity; cytoplasmic DGK-zeta facilitates p53 protein degradation.Courtesy of Kaoru Goto
DGKs differ in their molecular structure, enzymatic activity, subcellular localization and binding partners. One DGK, DGK-zeta, contains both a nuclear localization signal and nuclear export signal (1), suggesting a shuttling between the nucleus and the cytoplasm. Morphological studies on animal tissues report that DGK-zeta predominantly localizes to the nucleus in some cell types, while it exhibits both nuclear and cytoplasmic localization in others. from the nucleus to the cytoplasm in hippocampal neurons under stress conditions such as transient ischemia and seizures, and appears to be involved in stress responses.
What roles are assigned to DGK-zeta in the nucleus and the cytoplasm? Current data suggest that nucleocytoplasmic shuttling of DGK-zeta in neurons has a dual effect: While decreased levels of nuclear DGK-zeta lead to suppression of p53 transcriptional activity, increased levels of cytoplasmic DGK-zeta facilitate p53 protein degradation. reveal that DGK-zeta associates with p53, and DGK-zeta deletion suppresses p53 transcriptional activity under basal and DNA-damage conditions (2). DGK-zeta association with p53 facilitates cytoplasmic degradation of p53 through the ubiquitin proteasome system, or UPS. DGK-zeta deficiency, therefore, results in increased p53 levels. showed that DGK-zeta is degraded after cytoplasmic translocation in neurons, leading to increased levels of p53 protein and aberrant cell cycle reentry. In all, the levels of cytoplasmic DGK-zeta may serve as a supressor for p53 by facilitating its degradation, thus suppressing its cytotoxic effects.
What are the potential effects of DGK-zeta on other transcription factors? Nuclear factor kappa-light-chain-enhancer of activated B cells, or NF-kappaB, for example, is important in numerous biological processes, particularly immune response. showed that DGK-zeta knockdown enhances the NF-kappaB pathway in response to inflammatory cytokines. DGK-zeta downregulation accelerates phosphorylation of the p65 subunit and its nuclear translocation, increasing NF-kappaB transactivation activity. suggest that DGK-zeta exerts a regulatory effect on p53 and NF-kappaB (3).
A key question is determining the molecular mechanisms exerted by DGK-zeta on p53 and NF-kappaB. We want to know whether DGK-zeta catalytic activity, which alters the balance of DAG and phosphatidic acid in the nucleus and cytoplasm, is essential. How DGK activity modulates transcriptional activities is not known, but it turns out that p53 degradation mediated through cytoplasmic DGK-zeta and UPS is not kinase activity–dependent. unexpected roles of DGK-zeta on transcription factors; there is much to learn about how lipid-metabolizing enzymes impact cellular signaling involved in transcriptional regulation.
References
1. Goto, K. & Kondo, H. Proc. Natl. Acad. Sci. USA.93, 11196-11201 (1996).
2. Tanaka, T. et al. J. Cell Sci.126, 2785-2797 (2013).
3. Tanaka, T. et al. Adv. Biol. Regul.60, 15-21 (2016).
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and weтАЩll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Transforming learning through innovation and collaboration
Neena Grover will receive the William C. Rose Award for Exemplary Contributions to Education at the 2025 ASBMB Annual Meeting, April 12тАУ15 in Chicago.

Guiding grocery carts to shape healthy habits
Robert тАЬNateтАЭ Helsley will receive the Walter A. Shaw Young Investigator in Lipid Research Award at the 2025 ASBMB Annual Meeting, April 12тАУ15 in Chicago.

Quantifying how proteins in microbe and host interact
тАЬTo develop better vaccines, we need new methods and a better understanding of the antibody responses that develop in immune individuals,тАЭ author Johan Malmström said.

Leading the charge for gender equity
Nicole Woitowich will receive the ASBMB Emerging Leadership Award at the 2025 ASBMB Annual meeting, April 12тАУ15 in Chicago.

CRISPR gene editing: Moving closer to home
With the first medical therapy approved, thereтАЩs a lot going on in the genome editing field, including the discovery of CRISPR-like DNA-snippers called Fanzors in an odd menagerie of eukaryotic critters.

Finding a missing piece for neurodegenerative disease research
Ursula Jakob and a team at the University of Michigan have found that the molecule polyphosphate could be what scientists call the тАЬmystery densityтАЭ inside fibrils associated with AlzheimerтАЩs, ParkinsonтАЩs and related conditions.


