Parched no more
In 1991, was stuck in the audience of a lecture at which the speaker was just droning on. To kill time, the dentist-biochemist began to sketch out an idea he had for treating dry-mouth syndrome. The idea came to fruition last year as .
 |
| Bruce Baum, a dentist–biochemist, retired from the National Institute of Dental and Craniofacial Research in 2011. |
Dry-mouth syndrome is common among patients who undergo radiation therapy for head-and-neck cancers; their salivary glands get damaged by the treatment and no longer produce saliva. Others with dry-mouth syndrome are perimenopausal women and people with Sjögren’s syndrome, a poorly understood disease. The sensation of a persistently dry mouth can be uncomfortable and affect quality of life. The patients can’t enjoy certain foods because they become hard to swallow and taste.
In 1982, Baum had set up a dry-mouth clinic at the National Institutes of Health in collaboration with oral surgeon Phil Fox. In the mid-1980s, he and Fox decided to test a drug called pilocarpine, which was being used to treat dry eyes, on patients who still had partially functional salivary glands. The drug worked by stimulating the functional portions of the glands to overproduce saliva, and it went on to become the commercial product Salagen.
But Salagen was useless in patients whose salivary glands were almost destroyed. These were mainly head-and-neck cancer patients who had undergone extensive radiation treatment. Baum was frustrated that he was unable to help those patients.
By 1990, gene therapy was getting increasing attention, and Baum was keeping an eye on the research. His former postdoctoral adviser, , then at the National Heart, Lung and Blood Institute and now at Weill Cornell Medical School, was investigating gene therapy for cystic fibrosis. Lungs bear many similarities to the salivary glands, so Baum figured that any breakthroughs in gene therapy for a lung disease might be extended to salivary glands.
By 1991, Crystal’s group had preliminary data that it could transfer the lacZ gene to rat lungs. Baum began to think about how he could transfer genes into salivary glands.
Then came the boring lecture. Baum, who was sitting next to an old friend at the talk, sketched his idea onto a napkin and showed it to his friend. His friend took a look and said it could work.
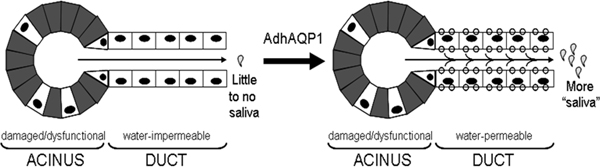
Baum’s idea was based on an old-fashioned dental technique. The largest of the major salivary glands are the pair of parotid glands. Each gland sits below an ear and looks like a bunch of grapes. Each gland has a straight pipe that runs through the cheek and empties in front of the first molar. Dentists have used this pipe to thread in a tiny tube to deliver contrast agents to the parotid glands for diagnostic X-rays. Baum considered using this same technique to deliver a viral vector carrying a gene to the parotid glands and to correct the dry-mouth defect. But the question remained: Which gene? came from at the Johns Hopkins School of Medicine, whose group had discovered aquaporin-1. Aquaporin-1 is a protein that naturally forms channels for water in the membranes of cells. Agre won the Nobel Prize in chemistry in 2003 for his discovery. “As soon as I became aware of his paper in the [Proceedings of the National Academy of Science], I called him up and we collaborated for a number of years,” says Baum.
Over the next 15 years, Baum and his collaborators successfully tested his idea on rats and miniature pigs. The parotid glands, as he intuited, were an ideal target for gene therapy. Thanks to the old-fashioned dental technique, they were easily accessible. There was no danger of a gene vector escaping from the glands and wreaking havoc elsewhere, because the parotid glands are covered in a tough, fibrous layer that forms a barrier between them and the circulatory system.
From the animal studies, Baum and his colleagues had sufficient evidence to get approval from the NIH and the U.S. Food and Drug Administration for human testing in 2008. They started a phase I clinical trial with 11 head-and-neck cancer survivors. The investigators infused the aquaporin-1 gene directly into one of the parotid glands. The gene was carried in a disabled, nonreplicating adenovirus.
Within the study’s first 42 days, five participants had increased levels of saliva secretion as well as a renewed sense of moisture and lubrication in their mouths. The six who didn’t benefit from gene therapy didn’t suffer serious side effects.
Two of the five patients who benefited from the treatment particularly struck Baum. One was a man who had complained that his parched mouth stopped him from enjoying his two favorite foods — macaroni and cheese and croutons in salad — because he would end up choking on them. After the first 14 days of the trial, the man’s saliva output started to increase, and he was thrilled to report that he could again eat both without difficulty. “He also said to us that he suddenly started drooling on his pillow again,” says Baum.
The other patient was a man who routinely participated in triathlons. “He complained it was just so hard for him to run because his mouth would be so dry from breathing through his mouth,” says Baum. But once the man started in the clinical trial, says Baum, he reported that “he didn’t have to use a water bottle during a race he ran. That was pretty impressive.”
Since his retirement from the National Institute of Dental and Craniofacial Research in 2011, Baum has let his colleagues move ahead with subsequent steps of the research. Four of the six patients who didn’t benefit from the trial had reactions to the adenovirus vector. The next step will be to try another vector. The patients for whom the adenoviral vector did not work are eligible to enroll for another trial with the different vector once the trial is approved.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Guiding grocery carts to shape healthy habits
Robert “Nate” Helsley will receive the Walter A. Shaw Young Investigator in Lipid Research Award at the 2025 ASBMB Annual Meeting, April 12–15 in Chicago.

Quantifying how proteins in microbe and host interact
“To develop better vaccines, we need new methods and a better understanding of the antibody responses that develop in immune individuals,” author Johan Malmström said.

Leading the charge for gender equity
Nicole Woitowich will receive the ASBMB Emerging Leadership Award at the 2025 ASBMB Annual meeting, April 12–15 in Chicago.

CRISPR gene editing: Moving closer to home
With the first medical therapy approved, there’s a lot going on in the genome editing field, including the discovery of CRISPR-like DNA-snippers called Fanzors in an odd menagerie of eukaryotic critters.

Finding a missing piece for neurodegenerative disease research
Ursula Jakob and a team at the University of Michigan have found that the molecule polyphosphate could be what scientists call the “mystery density” inside fibrils associated with Alzheimer’s, Parkinson’s and related conditions.

From the journals: JLR
Enzymes as a therapeutic target for liver disease. Role of AMPK in chronic liver disease Zebrafish as a model for retinal dysfunction. Read about the recent JLR papers on these topics.